The Repair study: Effects of macitentan on RV structure and function in pulmonary arterial hypertension1
VIEW THE SERAPHIN CLINICAL DATA to learn more about a large, outcomes-based pivotal trial in PAH.
Important Considerations1
- Data are based on a single-arm, open-label clinical trial and not on a randomized, placebo-controlled clinical trial
- CMR parameters have not been accepted as primary endpoints for pivotal studies in PAH, so these data are hypothesis generating and further research is needed for a better understanding of the significance of CMR parameters as proxy for disease progression
- The open-label design and study size of the Repair study limited subgroup analyses
- OPSUMIT® (macitentan) is an ERA indicated for the treatment of PAH (WHO Group I) (FC II-III) to reduce the risks of disease progression and hospitalization for PAH2
- This study was funded by Actelion Pharmaceuticals Ltd, a part of Johnson & Johnson Innovative Medicine
REPAIR was a 52-week, prospective, multicenter, single-arm, open-label, phase 4 study
Study objectives
- To evaluate the effect of OPSUMIT® on RV and hemodynamic properties in patients with PAH
- Primary endpoints were assessed at Week 26
- To evaluate the safety and tolerability of OPSUMIT® in patients with symptomatic PAH
- Patients’ median exposure time was 52 weeks
Inclusion criteria
- 18 to 74 years of age
- Idiopathic or heritable PAH, PAH related to CTD, drug use or toxin exposure, or simple congenital systemic-to-pulmonary shunts at least 2 years after repair (RHC required to confirm diagnosis)
- Patients who are PAH-treatment naïve or receiving a stable background PDE5 inhibitor for at least 3 months, have a 6MWD of ≥150 m, and a WHO FC I-III
Study population
- Primary interim analysis set: n=42*
- Final analysis set: n=71†‡
- Safety set: n=87§ǁ
Exclusion criteria¶
- Prior use of ERAs, stimulators of soluble guanylate cyclase, or prostacyclin/prostacyclin analogs
The primary endpoints in REPAIR were RVSV (assessed by CMR) and PVR (measured by RHC)1
| Primary endpoints: Change from baseline to Week 26 |
||
|---|---|---|
| RVSV assessed by CMR | ||
| PVR measured by RHC | ||
| Secondary endpoints: Change from baseline to Week 26 |
||
|---|---|---|
| RVESV, RVEDV, RVEF, and RV mass assessed by CMR | ||
| 6MWD | ||
| WHO FC | ||
The single-arm, open-label design and study size of the Repair study limited subgroup analyses.
Patient demographics1
Baseline characteristics (finals analysis set, n=71)
80.3%
(n=57)
45
years
I:
1.4%
(n=1)
II:
47.9%
(n=34)
III:
50.7%
(n=36)
411.2
meters
Etiology


Monotherapy and combination therapy
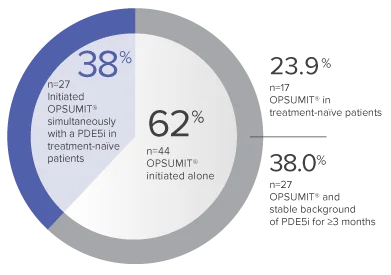
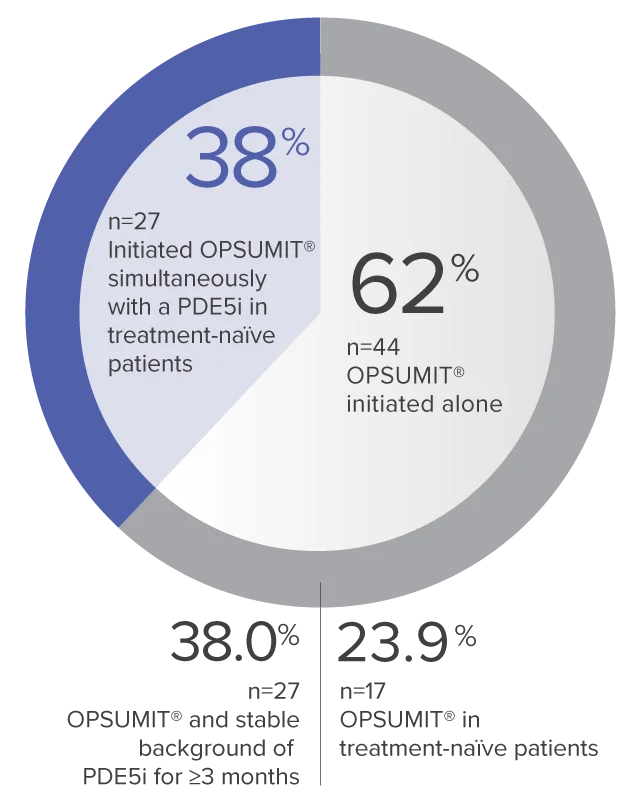
The single-arm, open-label design and study size of the Repair study limited subgroup analyses.
REPAIR Primary Endpoints: RVSV and PVR at Week 26
- The primary interim analysis set (n=42) was declared positive and enrollment was stopped at Week 26 as both primary endpoints (RVSV and PVR) were met
- The final analysis set (n=71) was consistent with the primary interim analysis set
Primary interim analysis set
(n=42)
RVSV
increase
Change from baseline
to Week 26††
LS mean (96% CL)
15.2 (9.3-21.0) mL
Baseline (mean±SD):
50.7±17.5 mL
PVR
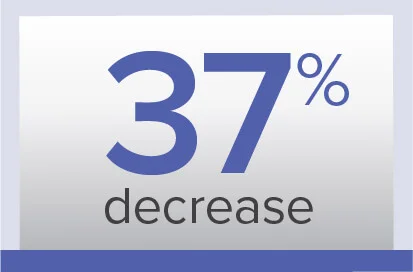
Week 26/baseline ratio‡‡
Geometric mean (99% CL)
0.63 (0.54-0.74) dyn·sec/cm5
Baseline (mean±SD):
900.2±457.6 dyn·sec/cm5
Final analysis set
(n=71)


RVSV
increase
Change from baseline
to Week 26††
LS mean (96% CL)
12.0 (8.4-15.6) mL
Baseline (mean±SD):
52.2±17.2 mL
PVR
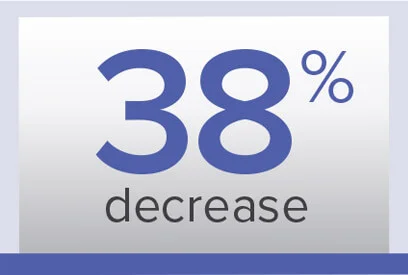
Week 26/baseline ratio‡‡
Geometric mean (99% CL)
0.62 (0.56-0.69) dyn·sec/cm5
Baseline (mean±SD):
974.6±679.0 dyn·sec/cm5
CMR parameters have not been accepted as primary endpoints for pivotal studies in PAH, so these data are hypothesis generating and further research is needed for a better understanding of the significance of CMR parameters as proxy for disease progression.
Observations of RV stroke volume, ejection fraction, and mass at Week 261§§
Final analysis set (n=70)
Change from baseline to Week 26‖‖ in RV parameters
LS mean (95% CL)
(mL)
–16.1
(–20.0 to –12.2)
Baseline (mean±SD):
90.2±40.6
(mL)
–6.2
(–12.8 to 0.4)
Baseline (mean±SD):
149.8±49.1
(%)
+10.6
(7.9 to 13.3)
Baseline (mean±SD):
37.7±14.3
(g)
–10.5
(–14.0 to –7.1)
Baseline (mean±SD):
110.4±47.5
Observations of 6MWD and WHO FC at Week 261
Exercise capacity at Week 26 (n=71)
| Baseline (mean±SD) |
Change from baseline to Week 26## LS mean (95% CL) |
|
|---|---|---|
| 6MWD (m) | 411.2±120.5 | 35.6 (19.0-52.3) |
FC at Week 26 (n=70)
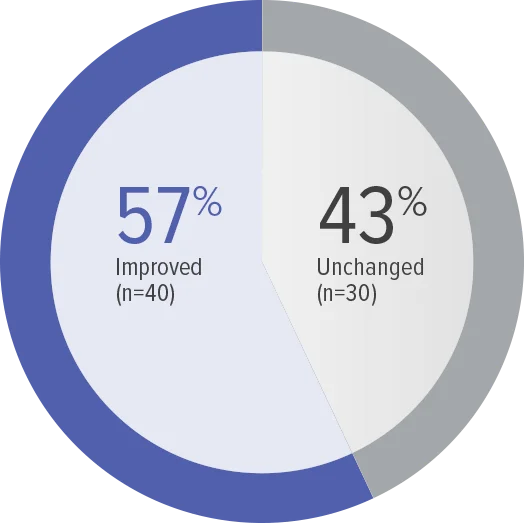
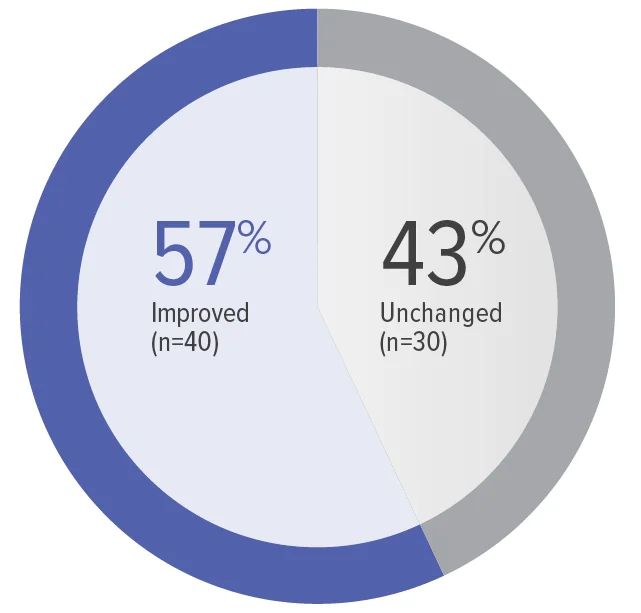
Zero patients worsened in WHO FC.
Baseline WHO FC#: WHO FC I, 1 (1.4%);
WHO FC II, 34 (48.6%); WHO FC III, 35 (50.0%).
Secondary efficacy analyses were performed with no correction for multiple testing; thus, these analyses are of an exploratory nature.
Safety and tolerability in the Repair study
Adverse events observed in the safety set (n=87)
Treatment-emergent adverse events
| Patients with ≥1 treatment-emergent adverse event in ≥10% of patients, n (%) | 75 (86.2) |
|---|---|
| Peripheral edema | 19 (21.8) |
| Headache | 18 (20.7) |
| Dizziness | 12 (13.8) |
| Cough | 10 (11.5) |
| Hemoglobin decreased | 10 (11.5) |
| Upper respiratory tract infection | 10 (11.5) |
| Myalgia | 9 (10.3) |
Adverse events leading to discontinuation of study treatment
| Patients with ≥1 adverse event leading to discontinuation of study treatment, n (%) | 7 (8.0) |
|---|---|
| Aspartate aminotransferase increased | 2 (2.3) |
| Transaminases increased | 2 (2.3) |
| Hypersensitivity | 1 (1.1) |
| Liver function test increased | 1 (1.1) |
| Edema peripheral | 1 (1.1) |
Select treatment-emergent serious adverse events***
| Patients with ≥1 treatment-emergent serious adverse event, n (%) | 14 (16.1) |
|---|---|
| Pneumonia | 3 (3.4) |
| Acute myocardial infarction | 2 (2.3) |
| Pulmonary arterial hypertension | 2 (2.3) |
| Pulmonary embolism | 2 (2.3) |
| Sepsis | 2 (2.3) |
| Cardiac arrest (1 death recorded was the result of a fatal cardiac arrest, which occurred after the patient experienced a pulmonary embolism) | 1 (1.1) |
- Laboratory abnormalities of ALT/AST ≥3 x the ULN were reported for 5 (5.8%) patients in the safety set1
7-Year Data
VIEW RESULTSSafety and Tolerability
LEARN MORE*Primary results were based on the interim analysis set: Prespecified set of the first 42 patients who received at least 1 dose of OPSUMIT® and had valid measurements for both primary endpoints at baseline and at Week 26.
†71 patients with both RVSV and PVR measures at baseline and Week 26.
‡Final analysis set: All enrolled patients who received at least 1 dose of OPSUMIT® and had valid measurement for both primary endpoints at baseline and at Week 26.
§87 patients received ≥1 dose of OPSUMIT®.
ǁSafety set: All screened patients who received at least 1 dose of OPSUMIT®.
¶Reference supplemental methods for a complete list of the exclusion criteria.
#OPSUMIT® is only indicated in WHO FC II and III.
**Only simple congenital systemic-to-pulmonary shunts at ≥2 years postsurgical repair.
††Adjusted change using an ANCOVA model with a factor for PAH-targeted background therapy and a covariate for baseline parameter value.
‡‡Adjusted change using an ANCOVA model with a factor for PAH-targeted background therapy and a covariate for baseline log PVR.
§§Not adjusted for multiplicity.
ǁǁAnalyzed using an ANCOVA with a factor for PAH-targeted background therapy and a covariate for baseline parameter value.
¶¶From pulmonary artery flow.
##From ANCOVA model on parameter change from baseline with factors for PAH-targeted treatment strategy, baseline WHO FC, and parameter at baseline as a covariate.
***Reference supplemental table 6 for a complete list of treatment-emergent serious adverse events.



