For U.S. Healthcare Professionals
PAH is a severe, progressive disease1
Pulmonary arterial hypertension (PAH) results from restricted flow through the pulmonary arteries, leading to increased pulmonary vascular resistance (PVR) and, ultimately, right heart failure and death.2
Hemodynamic trends in pulmonary arterial pressure (PAP), PVR, and cardiac output (CO) correlate with increasing disease severity.3 Early in the disease, PVR and PAP may be elevated, but due in part to right ventricular (RV) compensation, CO remains within normal range for an indeterminate time. Patients may not be diagnosed until they present with overt symptoms such as dyspnea, fatigue, and edema in the lower extremities.4
Learn AboutRisk assessment
PAH is associated with diverse pathologic events
The pulmonary arteries contract, and vascular resistance increases.3,4
Over time, the vascular intima thickens, further impeding blood flow.5
Chronic elevated pressures lead to right ventricular strain and overload.3,5
Vascular hypertrophy occurs in the presence of developing lesions and other arterial abnormalities.5,6
Abnormalities in platelet activation and function promote thrombosis and can lead to increased vasoconstriction.6
PAH is associated with diverse pathologic events
The pulmonary arteries contract, and vascular resistance increases.3,4
Chronic elevated pressures lead to right ventricular strain and overload.3,5
Over time, the vascular intima thickens, further impeding blood flow.5
Vascular hypertrophy occurs in the presence of developing lesions and other arterial abnormalities.5,6
Abnormalities in platelet activation and function promote thrombosis and can lead to increased vasoconstriction.6
A system out of balance
Endothelial dysfunction results in overproduction of vasoconstrictors such as endothelin-1 (ET-1) and underproduction of vasodilators such as nitric oxide and prostacyclin.4,5
ET-1 and its receptors (ETA and ETB) mediate a variety of deleterious effects such as vasoconstriction, fibrosis, proliferation, hypertrophy, and inflammation.7
Because 3 separate signaling pathways are involved in PAH, the use of combination therapy is a recommended treatment strategy.2
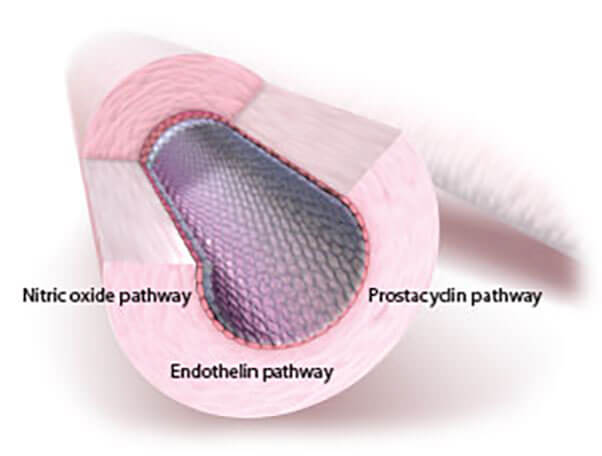
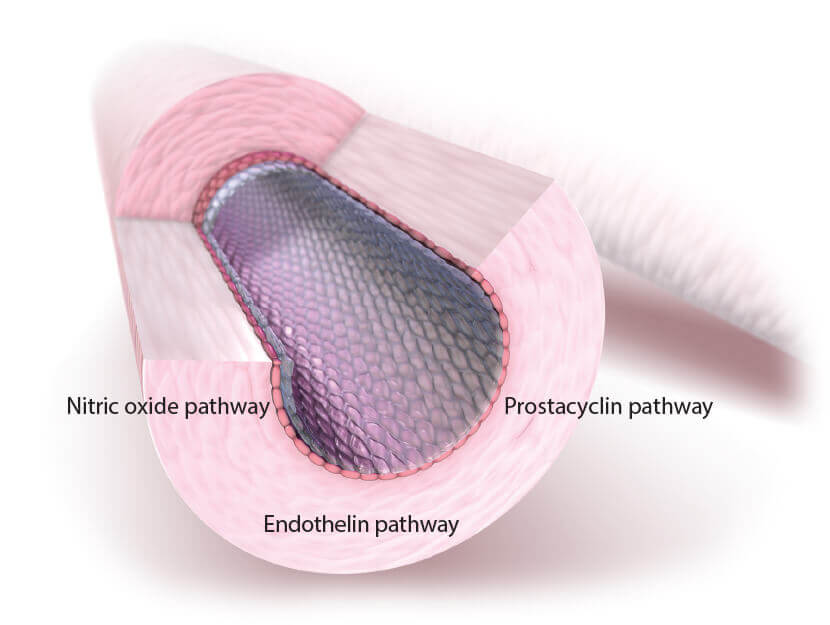
OPSUMIT® (macitentan) acts on the endothelin pathway, 1 of 3 principal pathways in PAH7,8
| Pathway | Drug Class |
|---|---|
| Endothelin | Endothelin receptor antagonists |
| Prostacyclin | Prostanoids |
| Nitric oxide | PDE-5 inhibitors, sGCs |
Risk assessment